•
Transfer of acquired products according to the FDA requirements
• Development of over 60 technologies for pharmaceutical dosage forms
• Process validation according to the EU and FDA requirements
• GMP implementation in pharmaceutical production plants
• Due diligence audit
• GMP compliance audit
• Suppliers audit
• Site Master File preparation
• Conceptual design for solid dosage forms, sterile and lyophilisation
plant, multi purpose drug factory, pharmaceutical warehouse, R&D
facility, QC laboratories etc.
• Strategic development studies, site development study
• Product development studies
• Marketing analyses
• Product registration
• Management of three new drug factory construction
• Numerous bidding procedures for pharmaceutical equipment clean rooms,
HVAC systems, laboratory equipment etc.
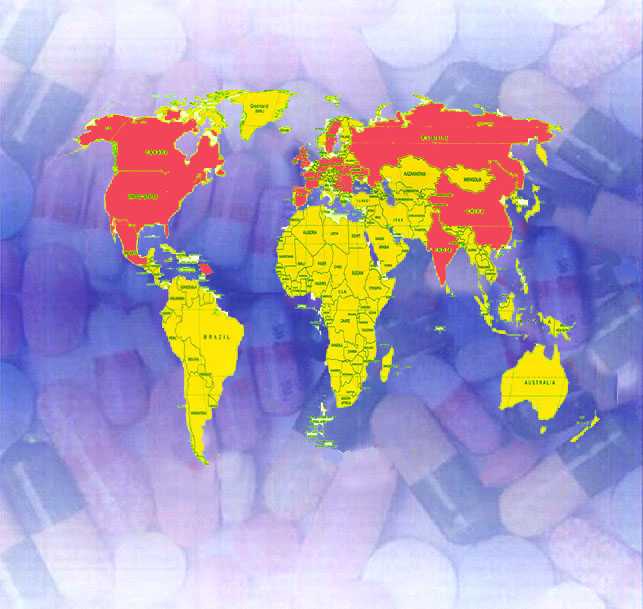
Working mainly for the big multinational company members of our consulting organization collected worldwide knowledge and experience in pharmaceutical industry. Countries in which members of our team have worked include:
• America (USA, Puerto Rico, Canada, Mexico)
• Western Europe (Spain, Switzerland, Scandinavia, UK, Ireland, Germany, France)
• Central Europe (Poland, Hungary)
• Eastern Europe (Russia, Yugoslavia, Macedonia, Ukraine, Bulgaria, Romania)
• Asia (China, India, Dubai, Bangladesh)